Answer:

Step-by-step explanation:
We are asked to find the temperature of a gas given a change in pressure and volume. We will use the Combined Gas Law, which combines 3 gas laws: Boyle's, Charles's, and Gay-Lussac's.
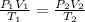
Initially, the gas has a pressure of 1.05 atmospheres, a volume of 4.25 cubic meters, and a temperature of 95.0 degrees Celsius.
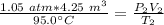
Then, the pressure increases to 1.58 atmospheres and the volume decreases to 2.46 cubic meters.
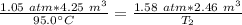
We are solving for the new temperature, so we must isolate the variable T₂. Cross multiply. Multiply the first numerator by the second denominator, then multiply the first denominator by the second numerator.
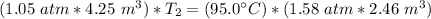
Now the variable is being multiplied by (1.05 atm * 4.25 m³). The inverse operation of multiplication is division, so we divide both sides by this value.
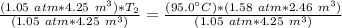
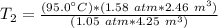
The units of atmospheres and cubic meters cancel.
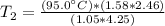
Solve inside the parentheses.

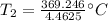
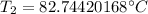
The original values of volume, temperature, and pressure all have 3 significant figures, so our answer must have the same. For the number we calculated, that is the tenths place. The 4 in the hundredth place to the right tells us to leave the 7 in the tenths place.
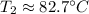
The temperature is approximately 82.7 degrees Celsius.