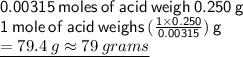Answer:
Molar mass of the unknown acid is 79 grams
Step-by-step explanation:
We have to first get moles in 15.0 ml of sodium hydroxide solution:
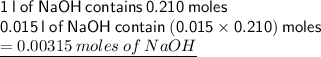
since mole ratio of acid : base is 1 : 1, so;
moles of acid that reacted is 0.00315 moles of the unknown acid.
then we've to get molar mass: