Answer:
Molar mass of the diprotic acid is 424 grams
Step-by-step explanation:
[hint: diprotic acid only contains 2 hydrogen protons]
Ionic equation:
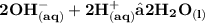
first, we get moles of potassium hydroxide in 28.94 ml :

since mole ratio of diprotic acid : base is 2 : 2, moles are the same.
Therefore, moles of acid that reacted are 0.008743 moles.
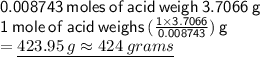
for the molar mass: