Answer:
Step-by-step explanation:
We are asked to calculate the mass of 2.75 moles of ammonia.
1. Molar Mass
We convert moles to grams using the molar mass or the mass of 1 mole of a substance. These values are equivalent to the atomic masses on the Periodic Table, but the units are grams per mole instead of atomic mass units.
Look up the individual elements in the compound of ammonia: NH₃
- Nitrogen: 14.007 g/mol
- Hydrogen: 1.008 g/mol
Notice that there is a subscript of 3 after H in the chemical formula. There are 3 moles of hydrogen in 1 mole of ammonia. We must multiply hydrogen's molar mass by 3 before adding nitrogen's molar mass.
- H₃: 1.008 * 3 = 3.024 g/mol
- NH₃: 14.007 + 3.024 = 17.031 g/mol
2. Convert Moles to Grams
We convert using dimensional analysis. First, set up a ratio using the molar mass.
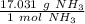
We are converting 2.75 moles of ammonia to grams, so we multiply the ratio by this value.
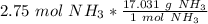
The units of moles of ammonia cancel.
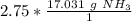
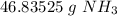
3. Round
The original measurement of moles (2.75) has 3 significant figures, so our answer must have the same. For the number we calculated, that is the tenth place. The 3 in the hundredth place tells us to leave the 8 in the tenth place.
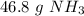
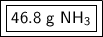
There are approximately 46.8 grams of ammonia in 2.75 moles of ammonia.