Answer:
A. 26 moles O2
B. 16 moles CO2
C. 20 moles H20
Step-by-step explanation:
a. 4 moles C4H10 x
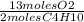 = 26 moles O2
= 26 moles O2
B. 4 moles C4H10 x
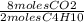 = 16 moles CO2
= 16 moles CO2
C. 4 moles C4H10 x
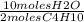 = 20 moles H20
= 20 moles H20
Use the coefficients of the balanced equation to make the convertions