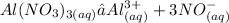Answer:
C. Aluminum Nitrate (Al(NO3)3)
Step-by-step explanation:
This is because, when Aluminium nitrate dissolves in water, it dissociates to form four ions ( one aluminium ion and three nitrate ions ). Since colligative property depends on number of particles or ions present ( roult's law ), this will create much effect.