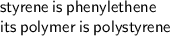Answer:
In styrene, there is a phenyl group which is electron-withdrawing. So the electronic density in the double bonds increases, hence easy to associate as monomers. While in methoxystyrene, there is a carbonyl group which is not electron deficient. so no easy association with monomers.
Step-by-step explanation: