Answer:
919,000 mg CCl4
Step-by-step explanation:
1 mole of any compound contains 6.023x10^23 molecules of that compound.
Use this relationship as a conversion factor: (6.022x10^23 molecules)/1 mole
(3.6 x 10^24 molecules of carbon tetrachloride)/((6.022x10^23 molecules)/1 mole) = 5.98 moles of carbon tetrachloride,
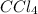
The molar mass of CCl4 is 153.8 grams/mole
(5.98 moles)(153.8 grams/mole) = 919 grams, or 919,000 mg CCl4