Answer:

Step-by-step explanation:
Molarity is a measure of concentration in moles per liter, therefore the formula is:
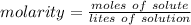
There are 2.0 moles of solute or sodium chloride dissolved in 0.25 liters of water or solution.
- moles of solute = 2.0 mol NaCl
- liters of solution = 0.25 L
Substitute these values into the formula.
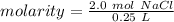
Divide.
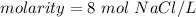
Molarity is measured in molars or M. 1 molar is equal to 1 mole per liter, so our answer is equal to 8 molars.
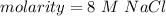
The original measurements of moles and liters have 2 significant figures, so our answer must have the same. For the number we calculated, that is the tenths place. There are no numbers to round, so we add a 0 in the tenths place.
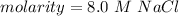
The molarity of the solution is 8.0 M NaCl.