Answer:

Step-by-step explanation:
We are asked to find the original volume of a gas given a change in temperature. Since pressure remains constant, we are only concerned with volume and temperature, so we use Charles's Law. This states the volume of a gas is directly proportional to the temperature. The formula for this law is:
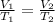
The gas begins with a temperature of 450 Kelvin, but the volume is unknown.
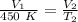
The gas is cooled to 248.9 Kelvin and the gas occupies a volume of 103.4 liters.
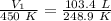
Since we are solving for the original volume, we must isolate the variable V₁. It is being divided by 450 Kelvin. The inverse operation of division is multiplication, so we multiply both sides of the equation by 450 K.
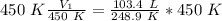
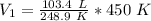
The units of Kelvin cancel.
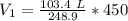
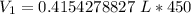
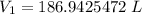
Round to the nearest hundredth. The 2 in the thousandths place tells us to leave the 4 in the hundredth place.
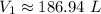
The original volume is approximately 186.94 liters and Choice A is correct.