Answer:
66.67%
Step-by-step explanation:
From the given information:
mass of cyclohexane = 2.9949 grams
density of cyclohexane = 0.779 g/mL
Recall that:
Density = mass/volume
∴
Volume = mass/density
So, the volume of cyclohexane = 2.9949 g/ 0.779 g/mL
= 3.8445 mL
Also,
mass of propylbenzene = 1.6575 grams
density of propylbenzene = 0.862 g/mL
Volume of propylbenzene = 1.6575 g/ 0.862 g/mL
= 1.9229 mL
The volume % composition of cyclohexane from the mixture is:
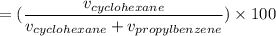
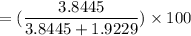
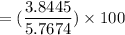
= 66.67%