Answer:
d. End product is that product with a ketone and carboxylic acid.
Step-by-step explanation:
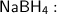
Sodium borohydride is a reducing agent, it reduces the ketone to a primary alcohol.

Then acidified water is an oxidising mixture which reverses the reduction reaction.