Answer:
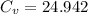 J / mol K
J / mol K
Step-by-step explanation:
Molar Heat Capacity
using the equipartition, the equation :
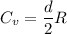
Here,
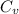 = molar heat capacity
= molar heat capacity
d = degree of freedom
R = Universal gas constant
Degree of freedom, d is 6
Universal gas constant, R = 8.317 J/ mol K
Therefore, the molar heat capacity is :
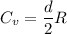
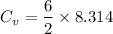
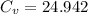 J / mol K
J / mol K