Question:-
Why it is difficult for haloarenes to undergo nucleoplhilic subsituⁿ reaction?
Answer:-
Haloarenes are less reactive towards the nucleoplhilic substitution rxⁿ . This is due to following reasons :-
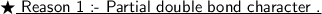
Halogen atom has one lone pair, and due to presence of π - σ - lp , resonance is established in the compound ( see attachment) . Due to resonance there is a partial double bond character in the carbon halogen bond , so it is difficult to break a double bond than a single bond.

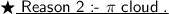
When a nucleoplhile comes to attack , it is repelled by the π-cloud of the benzene ring.

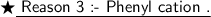
If somehow the halogen atoms leaves the benzene ring ,being more electronegative than carbon , it takes away the electron , thus a positive charge is left on benzene ring and the phenyl cation so formed is very unstable .
