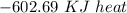Answer:
The right solution is "-602.69 KJ heat".
Step-by-step explanation:
According to the question,
The 100.0 g of carbon dioxide:
=
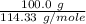
=
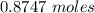
We know that 16 moles of
 formation associates with -11018 kJ of heat, then
formation associates with -11018 kJ of heat, then
0.8747 moles
 formation associates with,
formation associates with,
=
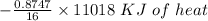
=
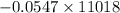
=