Answer:

Step-by-step explanation:
Molarity is a measure of concentration in moles per liter.
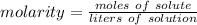
The solution contains 0.75 moles of sodium chloride and has a volume of 3.0 liters.
- moles of solute = 0.75 mol NaCl
- liters of solution = 3.0 L
Substitute these values into the formula.
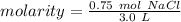
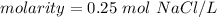
Molarity has the molar (M) as its unit. 1 molar is equal to 1 mole per liter.
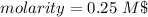
The molarity of the solution is 0.25 Molar and Choice D is correct.