Answer:
18.82 mg
Step-by-step explanation:
From the given information:
The molar mass of CO2 is calculated as follow
= (12 + (16 ×2))
= 44
The mass of carbon is determined by dividing the mass no of carbon from co2 by the molar mass of CO2, followed by multiplying it by 69.00 mg
=
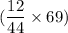
=(0.2727 × 69 )
= 18.82 mg