Answer:
The mass percentage composition of nitrogen in the sample of the cleaning detergent is approximately 16.78%
Step-by-step explanation:
The given mass of the sample of the cleaning detergent, m₁ = 20.5 g
The mass of the ammonium hydroxide, NH₄OH in the detergent, m₂ = 8.61 g
The molar mass of NH₄OH = 35.04 g/mol
The molar mass of nitrogen, N = 14.01 g/mol
Therefore, the mass, m₃ of nitrogen, N, in 8.61 g of ammonium hydroxide, NH₄OH, is found as follows;
m₃ = (14.01/35.04) × 8.61 g = (402,087/118,800) g ≈ 3.44 g
The mass of nitrogen, N, in the ammonium hydroxide, NH₄OH, contained in the 20.5 g sample of the cleaning agent, m₃ ≈ 3.44 grams
The percentage composition of nitrogen in the sample of the cleaning detergent, %N is given as follows;
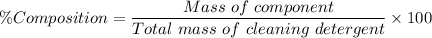
Therefore;
%N ≈ ((3.44 g)/(20.5 g)) × 100 ≈ 16.78 %
The percentage composition of nitrogen, %N ≈ 16.78%.