Step-by-step explanation:
The given data is:
The mass of hydrogen is 6.54 kg.
The actual yield is 30.4 kg.
The balanced chemical equation of the reaction is:
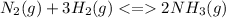
At first the theoretical yield should be calculated by using the balanced chemical equation:
3 mol. of hydrogen forms ---- 2 mol. of ammonia.
The molar mass of hydrogen is 2.0 g/mol.
The molar mass of ammonia is 17.0 g/mol.
Hence, the above statement can be rewritten as:
6g of hydrogen forms --- 34g of ammonia.
Then,
6.54g of hydrogen forms :
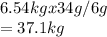
% yield = (actual yield /theoretical yield )x 100
=(30.4 kg /37.1 kg )x100
=81.9
Hence, % yield is 81.9.