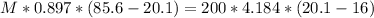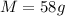Complete Question
A sample of aluminum, which has a specific heat capacity of 0.897 JB loc ! is put into a calorimeter (see sketch at right) that contains 200.0 g of water. The aluminum sample starts off at 85.6 °C and the temperature of the water starts off at 16.0 °C. When the temperature of the water stops changing it's 20.1 °C. The pressure remains constant at 1 atm. Calculate the mass of the aluminum sample.
Answer:
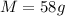
Step-by-step explanation:
From the question we are told that:
Heat Capacity
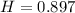
Mass of water
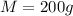
Initial Temperature of Aluminium
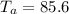
Initial Temperature of Water
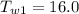
Final Temperature of Water
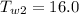
Generally
Heat loss=Heat Gain
Therefore