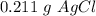Answer:
The answer is:
(a)
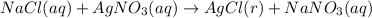
(b) NaCl
(c) 0.211 g
Step-by-step explanation:
Given:
The mass of NaCl,
= 0.0860 g
The molar mass of NaCl,
= 58.44 g/mol
The volume of
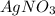 ,
,
= 30.0 ml
or,
= 0.030 L
Molarity of
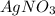 ,
,
= 0.050 M
Moles of NaCl will be:
=
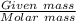
=
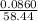
=
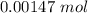
now,
Moles of
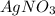 will be:
will be:
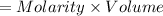
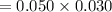
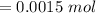
(a)
The reaction is:
⇒
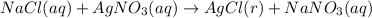
(b)
1 mole of NaCl react with,
= 1 mol of
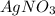
0.0015 mol
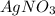 needs,
needs,
=
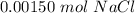
Available mol of NaCl < needed amount of NaCl
So,
The limiting reagent is "NaCl".
(c)
The precipitate formed,
=
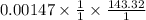
=