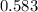Answer:
"0.583" is the appropriate answer.
Step-by-step explanation:
Let,
The initial constant of
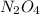 be "C".
be "C".
Amount of
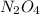 dissociated into
dissociated into
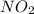 be "x".
be "x".
now,
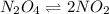
Initial constant C -
Equilibrium constant C 2x
The Kc is given as:
⇒
![K_c = ([NO_2]^2)/([N_2O_4])](https://img.qammunity.org/2022/formulas/chemistry/high-school/hhbp43s0uvzx0ooe7qiompglty6p59h4r1.png)
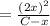
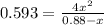
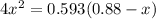
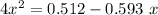
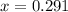
hence,
The constant of
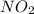 will be:
will be:
=
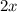
=