Answer:
The molar mass of gas Q is 43.923 g/mol
Step-by-step explanation:
The given volume of ethane gas that diffuses through a porous plug in 100 seconds = 100 cm³
Therefore;
The rate of diffusion of ethane gas through the porous plug,
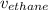 , is given as follows;
, is given as follows;
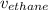 = (100 cm³/100 s) = 1 cm³/s
= (100 cm³/100 s) = 1 cm³/s
The molar mass of ethane, C₂H₆ = 2×12 g/mol + 6×1 g/mol = 30 g/mol
The given volume of gas, Q, that diffuses through a porous plug in 121 seconds = 100 cm³
∴ The rate of diffusion of the gas, Q,
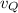 = 100/121 cm³/s
= 100/121 cm³/s
Graham's Law of diffusion states that the rate of diffusion of a gas is inversely proportional to the square root of the molecular mass of the gas
Mathematically, we have;
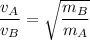
Where;
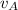 = The rate of diffusion of gas A
= The rate of diffusion of gas A
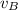 = The rate of diffusion of gas B
= The rate of diffusion of gas B
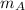 = The molar mass of the gas A
= The molar mass of the gas A
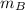 = The molar mass of the gas B
= The molar mass of the gas B
Therefore, for ethane and gas Q, measured under the same condition, we have;
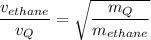
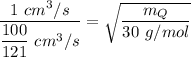
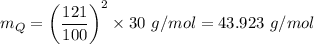
The molar mass of gas Q,
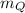 = 43.923 g/mol.
= 43.923 g/mol.