Answer:
a) The energy efficiency of the out-of-condition professor is 9.082 %.
b) The food calories needed by the well-conditioned athlete is 181.644 kilocalories.
Step-by-step explanation:
a) The energy efficiency of the food metabolization (
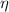 ), no unit, is defined by following formula:
), no unit, is defined by following formula:
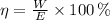 (1)
(1)
Where:
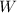 - Useful work, in joules.
- Useful work, in joules.
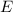 - Food energy, in joules.
- Food energy, in joules.
If we know that
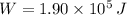 and
and
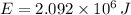 , the energy efficiency of the food metabolization is:
, the energy efficiency of the food metabolization is:
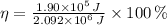
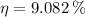
The energy efficiency of the out-of-condition professor is 9.082 %.
b) If we know that
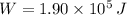 and
and
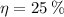 , then the quantity of food energy is:
, then the quantity of food energy is:
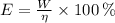
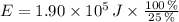
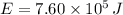
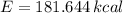
The food calories needed by the well-conditioned athlete is 181.644 kilocalories.