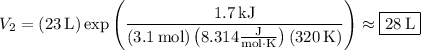From the ideal gas law,
PV = nRT ==> P = nRT/V
where P is the pressure exerted by the gas on the container. The work W done by this pressure as the volume of the gas changes from V₁ to V₂ is given by the integral,
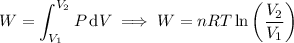
and solving for V₂ gives
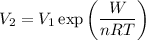
If you add 1.7 kJ of heat to the system, which does the aforementioned work, the gas will expand to a volume of