Step-by-step explanation:
The required concentration of
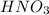 M1 =0.222 M.
M1 =0.222 M.
The required volume of
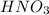 is V1 =225 mL.
is V1 =225 mL.
The standard solution of
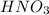 is M2 =16 M.
is M2 =16 M.
The volume of standard solution required can be calculated as shown below:
Since the number of moles of solute does not change on dilution.
The number of moles
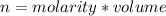
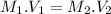
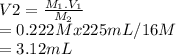
Hence, 3.12 mL of 16 m nitric acid is required to prepare 0.222 M and 225 mL of nitric acid.