Answer:

Step-by-step explanation:
We are asked to find how much of a 40 gram sample remains after 12 years.
Iron-55 has a half-life of 3 years. Therefore, after 12 years, 4 half-lives have been completed.
- 12 years/3 years = 4 half-lives
Every time a half-life is completed, half of the sample's mass decays. Remember we start with a 40 gram sample.
- 1 half- life: 40 g / 2 = 20 g
- 2 half-lives: 20 g / 2= 10 g
- 3 half-lives: 10 g / 2 = 5 g
- 4 half-lives: 5 g / 2 = 2.5 g
There is also a formula that can be used to solve this problem.
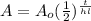
Where A₀ is the initial amount, t is the time, and hl is the half-life.
We know 40 grams is the inital amount, 12 years is the time, and 3 years is the halflife.
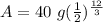
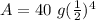
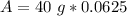
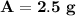
After 12 years, 2.5 grams of Iron-55 will remain.