Step-by-step explanation:
Molarity(M) of a solution is defined as the number of moles of solute(n) present in one liter of solution(V).
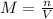
The number of moles(n) can be calculated as shown below:
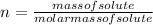
Molar mass of dextrose is 198.17 g/mol
Molar mass of NaCl is 58.5 g/mol.
Volume of the solution =250.0mL=0.250 L
The number of moels of dextrose(
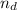 ) is:
) is:
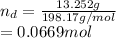
The number of moles of NaCl is:
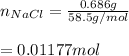
Thus, the molarity of dextrose is:
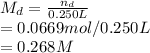
The molarity of NaCl is:
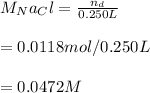
Answer:
The molarity of dextrose is 0.268 M.
The molarity of NaCl is 0.0472 M.