Answer:
A). 2.1 ×
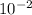
Step-by-step explanation:
Given reaction,
2
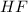 (g) ⇄
(g) ⇄
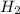 (g) +
(g) +
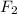 (g)
(g)
The concentrations are as following;
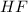 = 5.82 ×
= 5.82 ×
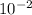 M
M
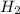 = 8.4 ×
= 8.4 ×
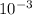 M
M
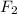 = 8.4 ×
= 8.4 ×
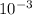 M
M
So,
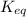 = [(
= [(
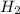 ) × (
) × (
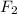 )] ÷ [
)] ÷ [
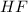 ]^2
]^2
Now,
We can determine the value of
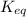 by substituting the values in above formula:
by substituting the values in above formula:
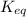 = [ (8.4 ×
= [ (8.4 ×
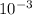 M) × (8.4 ×
M) × (8.4 ×
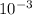 M)] ÷ [(5.82 ×
M)] ÷ [(5.82 ×
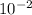 )^2
)^2
= 2.08 *
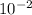
= 2.1 ×
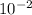
∵
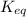 = 2.1 ×
= 2.1 ×
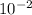
Thus, option A is the correct answer.