Solution :
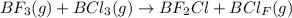
Explanation 1 :
Spontaneity of the reaction is based on two factors :
-- the tendency to acquire a state of minimum energy
-- the energy of a system to acquire a maximum randomness.
Now, since there isn't much difference in the bond enthalpies of B-F and B-Cl. So, we can say the major driving factor is tendency to acquire a state of maximum randomness.
Explanation 2 :
A system containing the
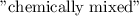 B halides has a
B halides has a
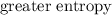 than a system of
than a system of
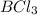 and
and
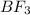 .
.
It has the same number of
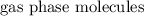 , but more distinguishable kinds of
, but more distinguishable kinds of
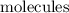 , hence, more microstates and higher entropy.
, hence, more microstates and higher entropy.