Answer:

Step-by-step explanation:
We are asked to find the new temperature of a gas after a change in volume. We will use Charles's Law, which states the volume of a gas is directly proportional to the temperature. The formula for this law is:
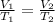
The volume is initially 52.3 liters at a temperature of 273 Kelvin.
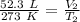
The volume reaches 145.7 liters at an unknown temperature.
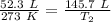
We are solving for the new temperature, so we must isolate the variable T₂. Cross multiply. Multiply the first numerator and second denominator, then the first denominator and second numerator.
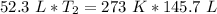
Now the variable is being multiplied by 52.3 liters. The inverse of multiplication is division. Divide both sides by 52.3 L.
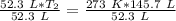
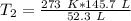
The units of liters cancel.
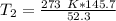
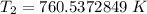
The original measurements have at least 3 significant figures, so our answer must have 3. For the number we found, that is the ones place. The 5 in the tenths place tells us to round the 0 up to a 1.
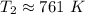
When the volume reaches 145.7 liters, the temperature is 761 Kelvin.