Step-by-step explanation:
The given data is:
The half-life of gentamicin is 1.5 hrs.
The reaction follows first-order kinetics.
The initial concentration of the reactants is 8.4 x 10-5 M.
The concentration of reactant after 8 hrs can be calculated as shown below:
The formula of the half-life of the first-order reaction is:
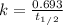
Where k = rate constant
t1/2=half-life
So, the rate constant k value is:
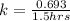
The expression for the rate constant is :
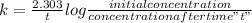
Substitute the given values and the k value in this formula to get the concentration of the reactant after time 8 hrs is shown below:
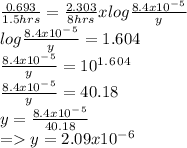
Answer: The concentration of reactant remains after 8 hours is 2.09x10^-6M.