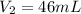Answer:
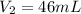
Step-by-step explanation:
From the question we are told that:
Pressure over Water
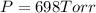
Temperature
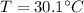
Pressure of Water
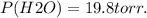
Volume of O2
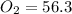
Pressure of Dry O2
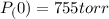
Generally the equation for Total Pressure is mathematically given by
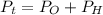
Therefore
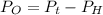
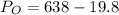
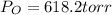
Generally the equation for Ideal gas is mathematically given by

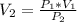
Therefore
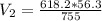
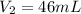
Hence,The volume would the dry O2 occupy at 755 torr