Answer:
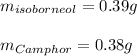
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to infer that the reaction whereby isoborneol goes to camphor occurs in a 1:1 mole ratio, that is why the theoretical yield of the latter is also 2.5 mmol (0.0025 mol) but the masses can be calculated as follows:
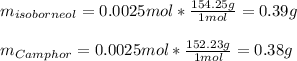
Because of the fact this is a rearrangement reaction whereas the number of atoms is not significantly modified.
Regards!