Answer:
There are 8Si atoms and 16 O atoms per unit cell
Step-by-step explanation:
From the question we are told that:
Edge length
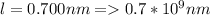
Density
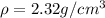
Generally the equation for Volume is mathematically given by
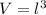
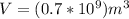

Where
Molar mass of (SiO2) for one formula unit
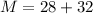
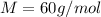
Therefore
Density of Si per unit length is
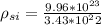
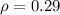
Molar mass of (SiO2) for one formula unit
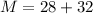
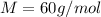
Therefore
There are 8Si atoms and 16 O atoms per unit cell