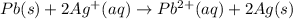Answer:
Following are the chemical equation to the given question:
Step-by-step explanation:
The Electrode is a silver film that is covered with such a thin coating of silver chloride, either by dipping its wire directly into silver-molten chloride, plating the wire using hydrogen peroxide, or oxidation silver in a chloride. In the given silver electrode, this anode acts as a cathode and thus reduces.
Half of the response reduction:
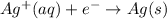
Half-effect oxidation:
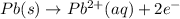
Complete reaction: