Step-by-step explanation:
Amount of water required in each case:
(a)The mass% of the solution is:9.95
Mass of solute that is urea is 6.80 g
To determine the mass of solvent water use the formula:
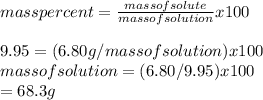
Hence the mass of solvent = mass of solution - the mass of solute
=68.3 g - 6.80g
=61.5 g
Hence, the answer is mass of solvent water required is 61.5 g.
(b) Given mass%=1.70
mass of solute MgBr2 = 29.3 g
The mass of solvent water required can be calculated as shown below:
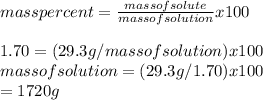
The mass of the solution is 1720 g.
Mass of solvent water = mass of solution - mass of solute
=1720 g - 29.3 g
=1690.7 g
Answer: The mass of water required is 1690.7 g.