Answer:
The temperature of the gas is 350.02 K.
Step-by-step explanation:
The average speed is related to the temperature as follows:
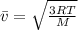 (1)
(1)
Where:
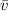 : is the average speed = 1477 m/s
: is the average speed = 1477 m/s
R: is the gas constant = 8.31 J/(K*mol)
T. is the temperature =?
M: is the molar mass
First, let's find the molar mass:
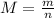
Where:
m: is the mass of the gas = 0.008 kg
n: is the number of moles = 2 mol
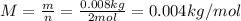
Hence, by solving equation (1) fot T we have:
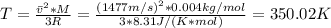
Therefore, the temperature of the gas is 350.02 K.
I hope it helps you!