Answer:
Option a is a solution acidic ([OH⁻] = 7.0x10⁻⁹ M).
Step-by-step explanation:
To know if a solution is acidic we need to calculate the pH and it must be lower than 7. A value of pH equal to 7 is a neutral solution and a solution with a pH value higher than 7 is a basic solution.
a. For the [OH⁻] = 7.0x10⁻⁹ M we have:
![pOH = -log[OH^(-)] = -log(7.0 \cdot 10^(-9)) = 8.15](https://img.qammunity.org/2022/formulas/chemistry/high-school/a25illb06nzyc104eesftxookjnunez5jx.png)
Now, the pH is:
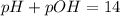
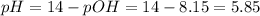
This solution is acidic (pH < 7)
b. [H₃O⁺] = 8.5x10⁻⁸ M
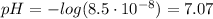
This is not an acidic solution. Is a neutral one (pH around to 7).
c. [OH⁻] = 2.5x10⁻⁶ M
![pOH = -log[OH^(-)] = -log(2.5 \cdot 10^(-6)) = 5.60](https://img.qammunity.org/2022/formulas/chemistry/high-school/fhwoox2b5a3i4fmdm0s8he4ahnmlpfq7bv.png)
Then, the pH is:
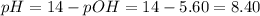
Hence, this is not an acidic solution. It is basic (pH > 7).
Therefore, option a is a solution acidic ([OH⁻] = 7.0x10⁻⁹ M).
I hope it helps you!