Answer:
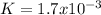
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to solve this problem by firstly setting up the equilibrium expression for the given reaction, in agreement to the law of mass action:
![K=([NO]^2)/([N_2][O_2])](https://img.qammunity.org/2022/formulas/chemistry/college/5ollyfxpi0del2q3p2j7kn0fqtfg1phq63.png)
Next, we plug in the given concentrations on the data table to obtain:
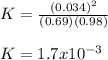
Regards!