Answer:
Step-by-step explanation:
We are asked to find the mass of helium gas given a volume.
1. Convert Liters to Moles
1 mole of any gas occupies a volume of 22.4 liters. So, 1 mole of helium gas contains 22.4 liters of helium. Create a ratio using this information.
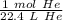
We are converting 15.3 liters of gas to moles, so we multiply by that value.
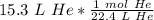
The units of liters of helium (L He) cancel.
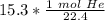
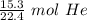
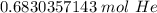
2. Convert Moles to Grams
The molar mass is the mass of 1 mole of a substance. These values are found on the Periodic Table as atomic masses, but the units are grams per moles. Look up the molar mass of helium.
Create another ratio using the molar mass.
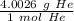
Multiply by the number of moles we calculated.
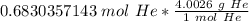
The units of moles of helium (mol He) cancel.
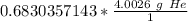
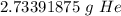
3. Round
The original measurement of volume has 3 significant figures, so our answer must have the same.
For the number we calculated, that is the hundredths place.
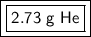
The 3 in the thousandths place to the right (2.73391875) tells us to leave the 3 in the hundredths place (2.73391875).
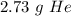
15.3 liters of helium gas is approximately 2.73 grams of helium.