Answer:
The number of hydrogen atoms is 4.96x10²⁴.
Step-by-step explanation:
The number of atoms can be found with the following equation:
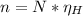
Where:
N: is the Avogadro's number = 6.022x10²³ atoms/mol
η: is the number of moles of hydrogen
n: is the number of hydrogen atoms
First, we need to find the number of hydrogen moles. The number of moles of CH₄ is:
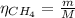
Where:
m: is the mass of methane = 33 g
M: is the molar mass of methane = 16.04 g/mol
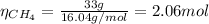
Now, since we have 4 hydrogen atoms in 1 mol of methane, the number of moles of hydrogen is:

Hence, the number of hydrogen atoms is:
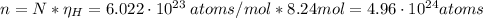
Therefore, the number of hydrogen atoms is 4.96x10²⁴.
I hope it helps you!