Answer:
1) 0.00498 mol Cu.
2) 0.00000374 mol CO₂
Step-by-step explanation:
Question 1)
We want to convert 3.00 * 10²¹ copper atoms into moles. Note that 3.00 is three significant figures.
Recall that by definition, one mole of a substance has exactly 6.022 * 10²³ amount of that substance. In other words, we have the ratio:
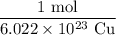
We are given 3.00 * 10²¹ Cu. To cancel out the Cu, we can multiply it by our above ratio with Cu in the denominator. Hence:
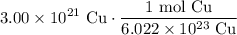
Cancel like terms:
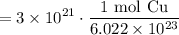
Simplify:
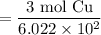
Use a calculator:
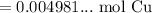
Since the resulting answer must have three significant figures:
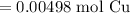
So, 3.00 * 10²¹ copper atoms is equivalent to approximately 0.00498 moles of copper.
Question 2)
We want to convert 2.25 * 10¹⁸ molecules of carbon dioxide into moles. Note that 2.25 is three significant digits.
By definition, there will be 6.022 * 10²³ carbon dioxide molecules in one mole of carbon dioxide. Hence:
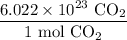
To cancel the carbon dioxide from 2.25 * 10¹⁸, we can multiply it by the above ratio with the carbon dioxide in the denominator. Hence:
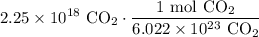
Cancel like terms:
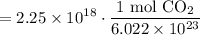
Simplify:
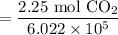
Use a calculator:
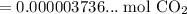
Since the resulting answer must have three significant figures:
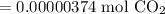
So, 2.25 * 10¹⁸ molecules of carbon dioxide is equivalent to approximately 0.00000374 moles of carbon dioxide.