❃ The following points should be kept in mind to write and balance a chemical equation :
Step 1 : Write the molecular formula of all the reactants and products correctly.
Step 2 : Separate reactants and products by a sign of arrow. If reactants or products are more than one, connect them by a sign of a plus.
Step 3 : Balance the atoms of O and H at last [ The atoms used at many places in an equation should be balanced at last ]. For balancing , the number should be added as coefficient i.e in the front of the molecules.
[ Remember those substance that take part in a chemical reaction are called reactants. Likewise , those substances which are formed after a chemical reaction are called products ]
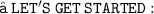
1. Carbon disulfide + Oxygen gas gives carbon dioxide + Sulfur dioxide.
Step 1 : The molecular formula of carbon disulfide is CS₂ , molecular formula of Oxygen gas is 0₂ [ Since oxygen is a diatomic element ] molecular formula of carbon dioxide is CO₂ and molecular formula of sulfur dioxide is SO₂.
Step 2 : CS₂ + O₂ ⟶ CO₂ + SO₂
Step 3 : In the reactant side , there is two ' S ' but on the other side , there is one ' S '. So , add 2 as a coefficient before S on the product side. Now , There are two ' O ' in the reactant side but six ' O ' in the product side. So , add 3 as a coefficient before O on the reactant side. Now , there are equal atom of C , S and O on both sides
i.e CS₂ + 3O₂ ⟶ CO₂ + 2SO₂
Answer :

-----------------------------------------
2. Silver + nitric acid gives silver nitrate + nitrogen dioxide + water
Step 1 : The molecular formula of Silver is Ag, molecular formula of nitric acid is HNO₃ , molecular formula of Silver nitrate is Ag ( No₃ ) , molecular formula of nitrogen dioxide is NO₂ and molecular formula of water is H₂O.
Step 2 : Ag + HNO₃ ⟶ Ag ( NO₃ ) + NO₂ + H₂O
Step 3 : In the reactant side , There is one ' H ' but on the other side , there are two ' H '. Now add 2 before H on the reactant side. There are equal atom of ' Ag ' , ' H ' , ' N ' , and ' O '.
i.e Ag + 2HNO₃ ⟶ Ag ( NO₃ ) + NO₂ + H₂O
Answer :
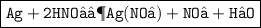
- The last step is a bit more confusing I guess. So , which balancing , count the atoms in following ways :
- The number written at the right lower corner of an atom is counted for that atom only. For example : In MgSO₄ , there are one ' Mg ' , one ' S ' and four ' O '
- The number written at the right lower corner of a bracket is for all the atoms enclosed in the bracket. For example : In Al₂ ( SiO₃ ) has two Al , three ' S ' and nine ' O '.
- The coefficient number is for all the atoms of the molecule. For example , in 2 Al ₂( SiO₃ )₃ , there are four ' Al ' , six ' Al ' and eighteen ' O '.
- Hope this helps! :)