e) The heating of sodium carbonate to produces sodium oxide and carbon dioxide
Solution given:
Balanced chemical equation:
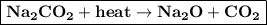
when sodium carbonate is heated it decomposed and form sodium oxide and carbon dioxide
f) chromium (III) hydroxide plus sulfuric acid
Balanced chemical equation:
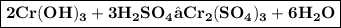
When chromium (III) hydroxide react with sulfuric acid double displacement takes place and forms produce chromium(III) sulfate and water.
g) aluminum metal plus chlorine gas
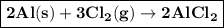
When aluminum metal is added to chlorine gas Combination reaction takes place and forms aluminum chloride.