Answer:
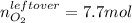
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out firstly necessary for us to set up the corresponding chemical equation:
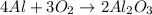
In such a way, we calculate the moles of aluminum consumed by 13.0 moles of oxygen in the reaction, by applying the 4:3 mole ratio between them:
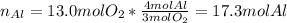
This means that Al is actually the limiting reactant and oxygen is in excess, for that reason we calculate the moles of oxygen consumed by 7.0 moles of aluminum:
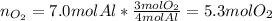
Thus, the leftover of oxygen is:
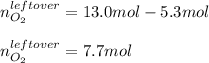
Whereas all the aluminum is assumed to be consumed.
Regards!