Answer: The concentration of fluoride ions after the addition of 9.00 mL of 0.0100 M HCl to 25.0 mL of this solution is 0.0709 M.
Step-by-step explanation:
Given: Concentration of hydrogen fluoride = 0.126 M
Concentration of fluoride ions = 0.1 M
Volume of HCl = 9.0 mL
Concentration of HCl = 0.01 M
Volume of HCl = 25.0 mL
Moles of
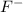 ions are calculated as follows.
ions are calculated as follows.
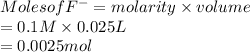
Moles of HF are as follows.
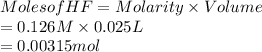
Moles of HCl are as follows.
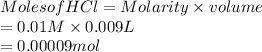
Now, reaction equation with initial and final moles will be as follows.
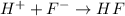
Initial: 0.00009 0.0025 0.00315
Equilibrium: (0.0025 - 0.00009) (0.00315 + 0.00009)
= 0.00241 = 0.00324
Total volume = (9.00 mL + 25.0 mL) = 34.0 mL = 0.034 L
Hence, concentration of fluoride ions is calculated as follows.
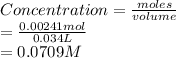
Thus, we can conclude that concentration of fluoride ions after the addition of 9.00 mL of 0.0100 M HCl to 25.0 mL of this solution is 0.0709 M.