Answer:
The answer is "5.18 ".
Explanation:
It's a question of chemistry. Therefore, the following solution is provided:
We will first determine the solution's pOH. The following can possible:
The concentration of Hydroxide ion
![[OH^(-)] = 1.5* 10^(-9)\ M](https://img.qammunity.org/2022/formulas/chemistry/high-school/14dkf769i04gndi74h6t13egm5fscludba.png)
![pOH =?\\\\pOH = -\log [OH^(-)]\\\\pOH = -\log 1.5* 10^(-9)\\\\pOH = 8.82\\\\](https://img.qammunity.org/2022/formulas/chemistry/high-school/dixtp7reatwg2jb43j77zsvp2dh5sbrhgc.png)
Furthermore, the pH of the solution shall be established. It was provided as follows:
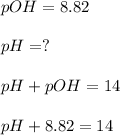
When collecting all the like terms:
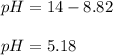
Therefore, the solution of pH is 5.18.