Answer:
Following are the solution to the given question:
Step-by-step explanation:
In the aqueous solution of
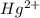 ion is used to represent a
ion is used to represent a
![[Hg(H_2O)_4]^(2+)](https://img.qammunity.org/2022/formulas/chemistry/college/m7zo1lwkggisjlxvxaz8pl60ftzl0acwdz.png) .
.
It is used to form a complex with iodide ions as
![[Hgl_4]^(2-)](https://img.qammunity.org/2022/formulas/chemistry/college/7rqy50lqlvhvadmdrlnxrwpumks0mrv5ej.png) .
.
Conversion reaction:
![[Hg(H_2O)_4]^(2+) +4I^(-) \to [HgI_4]^(2-)+4H_20](https://img.qammunity.org/2022/formulas/chemistry/college/kvj8cr4yzgljaayci67e1zly8gil6c78e7.png)
Following are the formation constants:
![k_f=([HgI_4]^(2-))/([Hg(H_2O)_4]^(2+) [I^(-)]^4)](https://img.qammunity.org/2022/formulas/chemistry/college/adhwzlaajmw1d5e963soygss9nid6d3hax.png)
Following are the first step to the conversion:
![[Hg(H_2O)_4]^(2+) +I^(-) \to [HgI(H_2O)_3]^(+)+H_20](https://img.qammunity.org/2022/formulas/chemistry/college/j6w59oafjkzzfwac4vdmad0vfz22c8c4qy.png)