Answer:
3
Step-by-step explanation:
Applying,
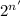 = R/R'............... Equation 1
= R/R'............... Equation 1
Where n' = number of halflives that have passed, R = Original atom of the substance, R' = atom of the substance left after decay.
From the question,
Given: R = 40 atoms, R' = 5 atoms
Substitute these values into equation 1
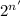 = 40/5
= 40/5
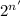 = 8
= 8
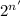 = 2³
= 2³
Equation the base,
n' = 3